The faculty advisor’s planning is essential to the successful completion of the IRB process for their student researchers. This webpage provides you, the faculty advisor, with tips and tools for supporting your students in conducting human subjects research.
Click here to download a printable Faculty Advisor Checklist to assist you with the IRB process.
Submission and Review Timeline
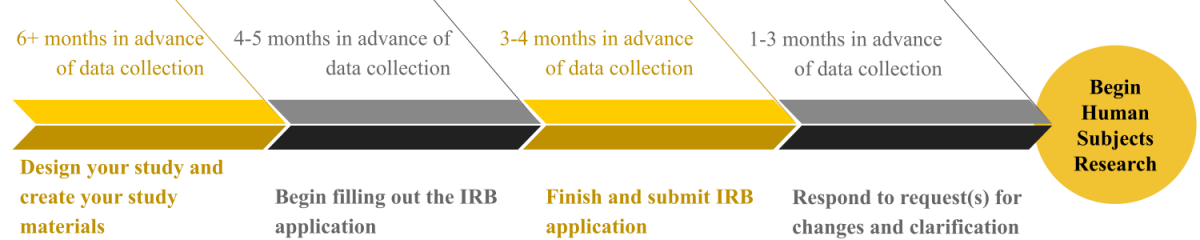
**If you have questions or concerns regarding this timeline, please reach out to the IRB office at IRB@appstate.edu. It is important to address timeline concerns at the beginning of the application process.**
Design Research Project Materials
Aprrox. 6 Months Before Starting Research
- Begin designing the research project with your student at least six months in advance of when you intend to begin data collection.
- Ensure that both you and your student have up-to-date CITI certifications. If either of you do not have active certifications, start working on completing one of the accepted courses (see here for more information). You must have up-to-date CITI certification to engage in human subjects research.
- Note: The information provided in the CITI Program courses is very helpful for designing ethical human subjects research and composing high quality IRB applications. Therefore, we recommend that all investigators complete CITI training before starting to draft your IRB application.
- Review the information on the Student Corner of the IRB website with your student. This will help you determine if an IRB application is necessary for the project, and will assist understanding the IRB application process.
- After you work with your student to identify the research question, work with them to identify the appropriate population and methodology for the research. This includes identifying and drafting the data collection instruments (e.g. surveys, interview questions, observation guides, etc.) that will be used in the research.
- Once the study design has been finalized and the study instruments have been identified and, where applicable, drafted, draft an appropriate informed consent process for the research. Guidance and templates for writing an informed consent form can be found on the Consent Corner of the IRB website.
- If desired, email irb@appstate.edu to schedule a pre-submission consultation with IRB staff. Note: The Faculty Advisor must schedule and attend all meetings with IRB staff.
Fill Out IRB Application
Approx. 4-5 Months Before Starting Research
- Both the student researcher and the faculty advisor must be listed as personnel on the application. The Principal Investigator (PI) must be a faculty member.
- The IRB application must include a complete description of all study procedures, all data collection instruments that will be used in the research, and the appropriate informed consent document. All study materials and procedures should be fully developed before your IRB application is submitted. Approval cannot be given for a partially drafted application or an application that is missing materials.
- All materials that will be presented to the participant as part of the research must be included in the IRB application. In addition, data collection instruments such as surveys, all recruitment materials, all handouts and instructions that will be given to participants, all scheduled communications (e.g., phone script, email script) with participants during the study, etc, must be included in your application.
Submit IRB Application and Seek Non-IRB Approvals
Approx. 3-4 Months Before Starting Research
- You, as the Faculty PI, have to initiate the IRB application process by following these instructions.
- You and your student should plan to submit the application the semester before you intend to begin data collection (i.e. mid-Spring semester for a project that will be conducted in the Fall; and early in the Fall semester for a project that will begin in the Spring). The earlier you submit your application, the more likely you are to receive IRB approval or exemption in time to begin data collection at the desired start date.
- Before the IRB Office can receive your application for review, you, the Faculty PI, must certify the completed submission in the system. Before clicking “Certify”, you, the Faculty PI, must first review your application to ensure that it is internally consistent. If the IRB application is for a thesis, it must match your student’s approved proposal.
- During this time, it is essential that you and your student familiarize yourself with all processes, procedures, rules, and regulations that apply to your research beyond the need for IRB review.
- If any research procedures will be conducted out of the country or if any research instruments or data will be imported or exported, please contact Export Controls to find out what you and your student will need to do to ensure compliance with the Export Controls requirements.
- Additionally, if the research will be conducted off-campus, it is essential that you and your student familiarize yourself with all the policies and laws that govern your specific research site. Many research sites (in particular, public and private schools) require additional site review and approval before recruitment can begin in that location. It is ultimately the Faculty PI’s responsibility to be aware of and compliant with all rules and restrictions that apply to the research site.
- Finally, if policies and regulations such as FERPA, HIPAA, PPRA, or state and local laws (e.g., NC Senate Bill 49---commonly known as the “Parents’ Bill of Rights”) apply to your research, it is your responsibility as the Faculty PI to ensure that you and your student are aware of these laws and regulations, and that the research procedures are conducted wholly in compliance with all applicable laws, ordinances, rules, and regulations.
Review Process and Required Revisions
Approx 1-3 Months Before Starting Research
- After reviewing your application, IRB staff will contact you if clarifications or changes are needed. Please resubmit the revised application in a timely manner so that the review can proceed.
- It is important to be prepared for multiple rounds of revisions.
- The initial stage of the review process (which often involves one or more rounds of revisions) is the technical review. During this process, IRB office staff members assist the research team with identifying and fixing inconsistencies, violations of university standard procedures, and concerns that would interfere with the application receiving IRB approval or exemption. This allows for a quicker and smoother final review process, and helps to ensure that the application received by the final reviewer(s) is in good condition. Technical reviews are a part of the review process for all levels of IRB review, including exempt research.
- Once the technical review process is complete and the application is ready to be scheduled for review, the submission is sent to a qualified member of the IRB or Research Protections staff for expedited or exempt review. If the study requires Full Board review, the study is scheduled for the following month’s IRB meeting. Please note that the application will not be scheduled for review until the technical review process has been completed, and the application is ready for review. This second part of the review process usually involves at least one round of required revisions, and these required revisions may result in different concerns than those identified by the technical reviewer. This should be expected, and is part of the structure of App State’s human subjects research review process.
- Addressing required changes to the IRB submission. It is the Faculty PI’s responsibility to address questions, concerns, and required changes identified in the returned IRB application. If you do not understand what is requested in the required stipulations, please email specific questions to irb@appstate.edu.
- If you have not received correspondence from the IRB office within 10 business days of submission (or resubmission), please feel free to email irb@appstate.edu for a status update. (Note: a round of review may take longer than 10 days, but we will be happy to provide you with a status update, if requested).
Final Steps
- Once the review process is complete, you will receive an email from Cayuse (the electronic submission system) with an Approval or Exemption Letter.
- Please note that you and your student are not permitted to begin any research procedures with human subjects (and are not permitted to start recruiting participants for the research) until your IRB application has received official approval or exemption.
- Once IRB approval or exemption has been obtained, and all additional approvals (including obtaining site permission, if applicable) have been obtained, you and your student may begin the research.
- The faculty PI is responsible for ensuring that the research is conducted in accordance with the approved or exempted application. If any changes need to be made to the approved research, you must submit a modification request following these instructions. You must receive an updated approval or exemption letter for the modified procedures and materials before the requested changes to the research may be implemented.
- If there is an adverse event or unanticipated problem related to the research, contact the IRB office immediately to receive instructions for how to proceed.
- Finally, after all research procedures are complete and all identifiers have been removed from the data, please submit a closure report in Cayuse.
Areas Where Students Require the Most Assistance
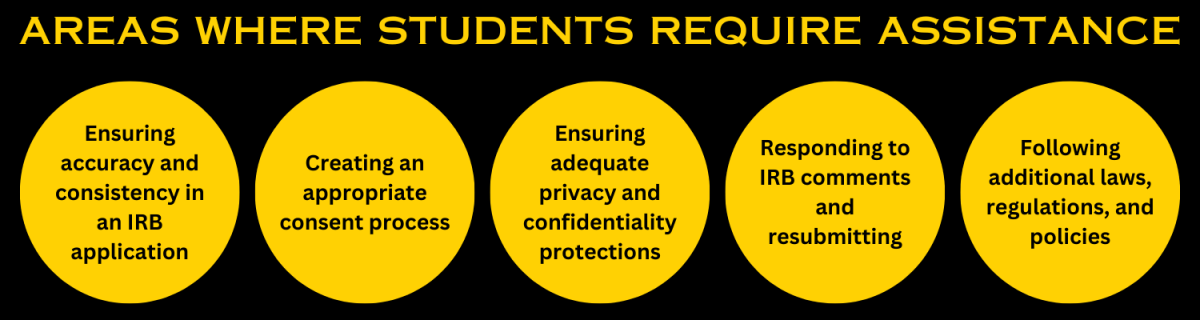
As faculty advisor for your student’s project, it is your responsibility to provide your student with the help and mentorship needed for their success. Additionally, as PI on the IRB submission, you are ultimately responsible for the accuracy of the application and the conduct of all aspects of the final research project. Therefore, it is very important for you, as the PI , to be involved at all stages of the IRB application process. The extent of your involvement will depend on the nature of the project and the experience of your student; you and your student should always work as a team throughout the IRB application process as well as conducting the research.
While some students will need assistance with all aspects of the application and research project, others will request much less assistance from their faculty advisors when designing their study and drafting the IRB application. Below, we have provided a list of areas of the study design and IRB application process where the greatest level of involvement from their faculty advisor is required. Under each heading, you’ll find suggestions and resources to help you and your students navigate each area of consideration.
Ensuring Accuracy and Consistency in an IRB Application
As faculty advisor, you are responsible for ensuring that the information in the IRB submission (where you are listed as Principal Investigator) is complete, accurate, and internally consistent. Please ensure that all documents uploaded are the final drafts of the documents you wish to use in the research, and that the project described in the IRB application matches the project that your student is approved to conduct, as described in their prospectus. Study design descriptions must include all necessary information required for a review, meaning that incomplete descriptions of procedures will always be sent back for clarification. Submitting a fully developed first draft of the IRB application will reduce the number of revisions required, and allow for a more expedient review timeline.
Creating an Appropriate Consent Process (including an appropriate consent form)
Exempt informed consent forms generally do not require signatures (unless the research includes information covered under FERPA). A common mistake is to include a signature line, even when signatures would be difficult or impossible to obtain (e.g. online surveys; Zoom interviews; etc). It is your responsibility to ensure that the entire informed consent process is appropriate for the proposed research study. If an informed consent form includes a signature line, adequate provisions must be in place for obtaining a signature from participants. A basic template for exempt research informed consent forms can be found on the Consent Corner webpage.
Non-exempt informed consent forms require signatures (unless written documentation of consent is specifically waived by the IRB). Additionally, there are several elements of informed consent that non-exempt informed consent forms must include to receive approval from the IRB. All of these elements are built into the non-exempt informed consent template found on the Consent Corner of the IRB website.
If biomedical procedures will be conducted as part of the research (including, but not limited to, physical examinations and exercise procedures), writing the informed consent forms will require care and attention to ensure plain language is used throughout. The IRB recommends that consent forms are written at the 6th-8th grade reading level. Guidance on writing in plain language can be found in the “Consent Guidance” section at the bottom of the Consent Corner webpage. If any element(s) of the consent form are missing from the informed consent form, the application will be returned with comments instructing you to add the missing information.
In accordance with federal regulations, informed consent forms for research are not permitted to include any language that waives or appears to waive any of the participants’ rights. Informed consent forms are not legally binding contracts and may not be written using contractual language. This means that you consent form may not request ownership of participants’ data, audio recordings, or visual images. The informed consent form is also never permitted to save harmless nor release the investigator, sponsor, or university from any liability. Please review the informed consent form to ensure that it is written in compliance with these requirements.
Finally, if the research will involve minors, you will need to draft appropriate parental permission and child assent materials. Please see the Consent Corner webpage for more information.
Ensuring Adequate Privacy and Confidentiality Protections
Conducting ethical human subjects research requires that appropriate privacy and confidentiality protections are put in place to protect participants. Attention will be needed to design adequate provisions for maintaining the privacy of participants and confidentiality of data.
Privacyrefers to an individual’s right to control access to their body and personal information and is primarily relevant during recruitment, the informed consent process, and during the collection of information and/or biospecimens. Examples of precautions that can be taken to protect privacy include: conducting the informed consent process and data collection procedures in a place where, aside from IRB approved members of the study team, no one else can see or overhear; if a Zoom focus group will occur as part of the research, encouraging participants to turn off their cameras and use a pseudonym; if Zoom or phone interviews will occur, asking participants to attend the interview in a private location where others cannot see or overhear; if survey procedures will be used, suggesting that participants take the survey in a location where others cannot see their responses; and if online survey procedures will be conducted, advising participants to take the survey using a private device and/or using private browsing mode (such as incognito mode in Google Chrome)
Confidentiality refers to the protection of data and biospecimens from unauthorized access. The risk of loss of confidentiality exists for all studies that involve the collection of identifiable data, even after the data collection procedures have been completed---this is why researchers are not permitted to submit a closure form until all identifiers have been destroyed. You and your student should both review ITS Office of Information Security’s Secure File Storage and Sharing guidance for information about how to protect the confidentiality of data collected for or used in the research.
Responding to IRB Comments and Resubmitting (During the Review Process)
As part of the IRB review process, the IRB application for your research project will likely require at least one round of revisions. When you receive notice that the IRB has returned your application with required revisions, please work diligently to make the required changes. Once all stipulations have been resolved and the resubmission has been completed, you, the Faculty PI, are required to certify the application before it can be received by the IRB Office for further review. Please note that the review of your study cannot continue until the IRB Office receives your revised application.
Following additional laws, regulations, policies, and restrictions related to the research
As the faculty PI of the IRB application, you are responsible for ensuring that you and your student are aware of any additional laws, regulations, policies, or requirements that apply to your research beyond the requirement for IRB review.
For research that involves students, FERPA, PPRA, and the NC Parents’ Bill of Rights may apply, and each poses additional restrictions that extend beyond the requirements of the federal regulations regulating human subjects research. Additionally, if the research is to be conducted in a classroom and/or specifically involves students or employees at educational institutions, the school (and possibly the school district) likely has additional unique policies and procedures that must be followed. Site permission is generally required to conduct research in schools, with some school districts requiring an additional review and approval processes that are distinct from the IRB approval process. In all likelihood, these additional processes will require additional time; plan accordingly. It is the responsibility of the research team to ensure that all relevant considerations associated with conducting research involving students or school employees are understood and taken into account when conducting the research.
For research that involves protected health information, it is the PI’s responsibility to ensure compliance with all applicable requirements of HIPAA.
Finally, if the research procedures will occur in a location other than on App State campus and/or if recruitment will focus on certain groups, members of organizations, and/or students or employees at particular institutions, it is the research team’s responsibility to ensure that all procedures are conducted in compliance with site policies and procedures. This may require obtaining site permission or undergoing additional reviews, as required by the research site, after receiving IRB approval or exemption at App State. In all likelihood, these additional processes will require additional time; plan accordingly.